20240827 Fullt upp!
Tjaaa vi ser fram emot att få vara sociala igen o inte uppslukade av underhåll bär o kommande frukt...
MASLOWS BEHOVSTRAPPA ENLIGT
GERD LUNDSTEN
Publicerad 20190514
Redigerad 20221209
Jag kommer egentligen ingen vart utan
Allergi-Överkänslighets-Säkrad-Miljö!
Detta innebär många gånger att saker o ting...
(Hmmm?...?...! Måste Tänka! Återkommer!)
DET VILL SÄGA:
AÖ-Säkrad-Miljö
Bla:
Fritt-Från: Djur- Rök- Parfym-/Irriterande Doftfritt!
Eliminationskost!
MASLOWS BEHOVSTRAPPA ENLIGT
GERD LUNDSTEN
Gå längst ned i inlägget o LÄS Er Uppåt!
Lyft!
7b ===•=====•=== Toppen på IsBerget...
Fritid Intressen
7a ===•=====•===
AÖ-Säkrad-Miljö
Bla:
Fritt-Från: Djur- Rök- Parfym-/Irriterande Dofter!
Eliminationskost!
6b ===•=====•===
Kläder Skor
6a ===•=====•===
AÖ-Säkrad-Miljö
Bla:
Fritt-Från: Djur- Rök- Parfym-/Irriterande Dofter!
Eliminationskost!
5b ===•=====•===
Arbete
5a ===•=====•===
Bla:
Fritt-Från: Djur- Rök- Parfym-/Irriterande Dofter!
Eliminationskost!
4b ===•=====•===
ArbetsRehab
4a ===•=====•===
AÖ-Säkrad-Miljö
Bla:
Fritt-Från: Djur- Rök- Parfym-/Irriterande Dofter!
Eliminationskost!
3b ===•=====•===
Trygghet Mat
Tak Sömn
Mediciner C-Pap
Kläder Skor
Sjukgymnastik
Arbetsterapi
3a ===•=====•===
AÖ-Säkrad-Miljö
Bla:
Fritt-Från: Djur- Rök- Parfym-/Irriterande Dofter!
Eliminationskost!
2b ===•=====•===
Tro Kärlek Vänskap
Gemenskap Samarbete Uppskattning
2a ===•=====•===
AÖ-Säkrad-Miljö
Bla:
Fritt-Från: Djur- Rök- Parfym-/Irriterande Dofter!
Eliminationskost!
1 ===•=====•===
AllergiÖverkänslighets(AÖ)-Säkrad-Miljö
Bla:
Fritt-Från: Djur- Rök- Parfym-/Irriterande Dofter!
Eliminationskost!
19/9-2017! NORRLANDSKLINIKEN!













Cyklade till nya simhallen NAVET! Det var första gången sedan jag blev dålig förra året.
Jag simmade 1000m. Dvs 20 längder i 50m-bassängen.
O jag gick runt o spanade in anläggningen. Personalen var som alltid mkt trevliga.
Jag cyklade hem o det fick lite trögt i början innan jag fått fart på benen. Det gick BRA att cykla. Ingen baksmälla från hjärta o lungor.
6/9-1016! Tisdag! Tränat!
Cyklat till NUS o sammanstrålat m min make Michael efter att han slutat jobbet.
Det gick bättre än förväntat att cykla. Jag cyklade tex på 2:ans vxl av 3 uppför den branta svingen. O nysningen o hjärtat fungerade mao bra. O andningsfrekvensen lade sig mkt fort.
Cyklade hem o det gick också mkt bra. I ett svep utan alla dom stopp jag hade förut.
|
Acute respiratory distress syndrome
|
|---|
| Classification and external res |
| J80 | |
| ICD-9 | 518.5, 518.82 |
|---|---|
| DiseasesDB | 892 |
| MedlinePlus | 000103 |
| eMedicine | article/165139 |
| Patient UK | Acute respiratory distress syndrome |
| MeSH | D012128 |
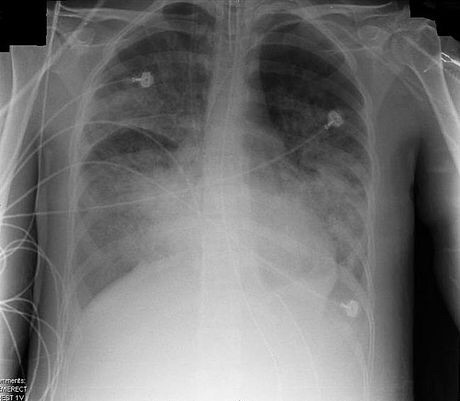
Acute respiratory distress syndrome (ARDS), previously known as respiratory distress syndrome (RDS), adult respiratory distress syndrome, or shock lung, is a severe, life-threatening medical condition characterized by widespread inflammation in the lungs. Whiles ARDS may be triggered by a trauma or lung infection, it is usually the result of sepsis.
ARDS is a disease of the lung tissue (alveoli) that leads to decreased exchange of oxygen and carbon dioxide (gas exchange). ARDS is associated with several pathologic changes: the release of inflammatory chemicals, breakdown of the cells lining the lung's blood vessels, surfactant loss leading to decreased surface tension in the lung, fluid accumulation in the lung, and excessive fibrous connective tissue formation.
The syndrome has a high mortality between 20 and 50%. The mortality rate with ARDS varies widely based on disease severity, patient age and the presence of other medical conditions.
The acronym ARDS formerly signified "adult respiratory distress syndrome" to differentiate it from "infant respiratory distress syndrome", which occurs in premature infants. However, as this type of pulmonary edema also occurs in children, ARDS has gradually shifted to mean "acute" rather than "adult". The differences from the typical infant syndrome remain.
ARDS is an acute injury to the lungs that results in the flooding of the alveoli, which are the small air sacs within the lungs where the exchange of oxygen and carbon dioxide occurs, partial collapse of the lungs (atelectasis) and low levels of oxygen in the blood (hypoxemia). However, in ARDS, these changes are not due to heart failure.
ARDS usually begins within 72 hours of the initial insult or injury to the lung, which may include sepsis, pneumonia, trauma, aspiration, etc,[1] and causes shortness of breath, fast breathing, and a low oxygen level in the blood.[2][3] A chest x-ray would show generalized infiltrates or opacities on both sides of the lungs (bilaterally),[1] which represent the fluid that accumulates in the lungs.
Other symptoms that occur in people with ARDS may be associated with the underlying disease process that has caused the ARDS. For example, a person with sepsis may have low blood pressure and fever, while a person with pneumonia may have a cough.
The predisposing factors of ARDS are numerous and varied. Sepsis, multiple blood transfusions, lung contusion, aspiration of gastric contents and drug abuse or overdose are common.[3] Also, burns, pancreatitis, smoke inhalation, pneumonia and near drowning can cause this condition. The inhalation of irritants, chemical warfare agents such as phosgene, chlorine gas and such can also cause ARDS.
Some cases of ARDS are linked to large volumes of fluid used during post-trauma resuscitation.[4] Other causes include shock, near-drowning and inhalation of irritants or toxic fumes that damage the alveolar epithelium.
The list of predisposing factors is extensive and some do not necessarily seem to have anything to do with injuring the lungs. Therefore, this syndrome is best diagnosed and managed on its criteria first, with retrospective management on whatever conditions may have precipitated it.
ARDS is characterized by the following criteria:[5][6]
(a minimum PEEP of 5 cmH
2O is required; it may be delivered noninvasively with CPAP to diagnose mild ARDS). A decreased PaO
2/FiO
2ratio indicates reduced arterial oxygen content relative to that of the inhaled gas, indicating a failure of the lung to transport oxygen into the blood.
The above characteristics are the "Berlin criteria" of 2012 by the European Society of Intensive Care Medicine, endorsed by the American Thoracic Society and the Society of Critical Care Medicine. They are a modification of the previously used criteria:[7][8]
An arterial blood gas analysis and chest X-ray allow formal diagnosis. Although severe hypoxemia is generally included, the appropriate threshold defining abnormal PaO
2 has never been systematically studied. A severe oxygenation defect is not synonymous with ventilatory support. Any PaO
2below 100 (generally saturation less than 100%) on a supplemental oxygen fraction of 50% meets criteria for ARDS. This can easily be achieved by high flow oxygen supplementation without ventilatory support. While CT scanning leads to more accurate images of the pulmonary parenchyma in ARDS, it has little use in the clinical management of patients with ARDS and remains largely a research tool.
ARDS is a clinical syndrome associated with pathological findings including pneumonia, eosinophilic pneumonia, cryptogenic organizing pneumonia, acute fibrinous organizing pneumonia, and diffuse alveolar damage (DAD). Of these, the pathology most commonly associated with ARDS is DAD, which is characterized by a diffuse inflammation of lung parenchyma. The triggering insult to the parenchyma usually results in an initial release of chemical signalsand other inflammatory mediators secreted by local epithelial and endothelial cells.
Neutrophils and some T-lymphocytesquickly migrate into the inflamed lung parenchyma and contribute in the amplification of the phenomenon. Typical histological presentation involves diffuse alveolar damage and hyaline membrane formation in alveolar walls. Although the triggering mechanisms are not completely understood, recent research has examined the role of inflammation and mechanical stress.
Inflammation, such as that caused by sepsis, causes endothelial dysfunction, fluid extravasation from the capillaries and impaired drainage of fluid from the lungs. Dysfunction of type II pulmonary epithelial cells may also be present, with a concomitant reduction in surfactantproduction. Elevated inspired oxygen concentration often becomes necessary at this stage, and may facilitate a 'respiratory burst' in immune cells. In a secondary phase, endothelial dysfunction causes cells and inflammatory exudate to enter the alveoli. This pulmonary edemaincreases the thickness of the alveolo-capillary space, increasing the distance the oxygen must diffuse to reach blood, which impairs gas exchange leading to hypoxia, increases the work of breathing and eventually induces fibrosis of the airspace.
Edema and decreased surfactant production by type II pneumocytes may cause whole alveoli to collapse or to completely flood. This loss of aerationcontributes further to the right-to-left shunt in ARDS. As the alveoli contain progressively less gas, the blood flowing through the alveolar capillaries is progressively less oxygenated, resulting in massive intrapulmonary shunting. Collapsed alveoli and small bronchi do not allow gas exchange. It is common to see patients with a PaO
2 of 60 mmHg (8.0 kPa) despite mechanical ventilation with 100% inspired oxygen.
The loss of aeration may follow different patterns depending upon the nature of the underlying disease and other factors. These are usually distributed to the lower lobes, in their posterior segments, and they roughly correspond to the initial infected area. In sepsis or trauma-induced ARDS, infiltrates are usually more patchy and diffuse. The posterior and basal segments are always more affected, but the distribution is even less homogeneous. Loss of aeration also causes important changes in lung mechanical properties that are fundamental in the process of inflammation amplification and progression to ARDS in mechanically ventilated patients.
Mechanical ventilation is an essential part of the treatment of ARDS. As loss of aeration and the underlying disease progress, the end tidal volume grows to a level incompatible with life. Thus, mechanical ventilation is initiated to relieve respiratory muscles of their work and to protect the usually obtunded patient's airways. However, mechanical ventilation may constitute a risk factor for the development—or the worsening—of ARDS.[7] Aside from the infectious complications arising from invasive ventilation with tracheal intubation, positive-pressure ventilation directly alters lung mechanics during ARDS. When these techniques are used the result is higher mortality through barotrauma.[7]
In 1998, Amato et al. published a paper showing substantial improvement in the outcome of patients ventilated with lower tidal volumes (Vt) (6 mL·kg−1).[7][9]This result was confirmed in a 2000 study sponsored by the NIH.[10] Both studies were widely criticized for several reasons, and the authors were not the first to experiment with lower-volume ventilation, but they increased the understanding of the relationship between mechanical ventilation and ARDS.
One opinion[who?] is that the forces applied to the lung by the ventilator may work as a lever to induce further damage to lung parenchyma. It appears that shear stress at the interfacebetween collapsed and aerated units may result in the breakdown of aerated units, which inflate asymmetrically due to the 'stickiness' of surrounding flooded alveoli. The fewer such interfaces around an alveolus, the lesser the stress. Even relatively low stress forces may induce signal transductionsystems at the cellular level, thus inducing the release of inflammatory mediators.[citation needed]
This form of stress is thought to be applied by the transpulmonary pressure(gradient) (Pl) generated by the ventilator or, better, its cyclical variations. The better outcome obtained in patients ventilated with lower Vt may be interpreted as a beneficial effect of the lower Pl. Transpulmonary pressure is an indirect function of the Vt setting on the ventilator, and only trial patients with plateau pressures (a surrogate for the actual Pl) were less than 32 cmH
2O(3.1 kPa) had improved survival.[citation needed]
The way Pl is applied on alveolar surface determines the shear stress to which lung units are exposed. ARDS is characterized by a usually inhomogeneous reduction of the airspace, and thus by a tendency towards higher Pl at the same Vt, and towards higher stress on less diseased units. The inhomogeneity of alveoli at different stages of disease is further increased by the gravitational gradient to which they are exposed and the different perfusion pressures at which blood flows through them. Finally, abdominal pressure exerts an additional pressure on inferoposterior lung segments, favoring compression and collapse of those units.[citation needed]
The different mechanical properties of alveoli in ARDS may be interpreted as having varying time constants—the product of alveolar compliance × resistance. A long time constant indicates an alveolus which opens slowly during tidal inflation, as a consequence of contrasting pressure around it, or altered water-air interface inside it—loss of surfactant, flooding.[citation needed]
Slow alveoli are said to be "kept open" using positive end-expiratory pressure, a feature of modern ventilators which maintains a positive airway pressure throughout the whole respiratory cycle. A higher mean pressure cycle-wide slows the collapse of diseased units, but it has to be weighed against the corresponding elevation in Pl/plateau pressure. Newer ventilatory approaches attempt to maximize mean airway pressure for its ability to "recruit" collapsed lung units while minimizing the shear stress caused by frequent openings and closings of aerated units. The prone position also reduces the inhomogeneity in alveolar time constants induced by gravity and edema. If clinically appropriate, mobilization of the ventilated patient can assist in achieving the same goal.[citation needed]
Mechanical ventilation can exacerbate the inflammatory response in patients with ARDS by including cyclic tidal alveolar hyperinflation and/or recruiting/derecruiting.[11] Stress index is measured during constant-flow assist-control mechanical ventilation without changing the baseline ventilatory pattern. Identifying the steadiest portion of the inspiratory flow (F) waveform fit the corresponding portion of the airway pressure (Paw) waveform in the following power equation:
Paw = a × tb + c where the coefficient b—the Stress Index—describes the shape of the curve. The Stress Index depict a constant compliance if the value is around 1, an increasing compliance during the inspiration if the value is below 1, and a decreasing compliance if the value is above 1. Ranieri, Grasso, et al. set a strategy guided by the stress index with the following rules:
Alveolar hyperinflation in patients with focal ARDS ventilated with the ARDSnet protocol is attenuated by a physiologic approach to PEEP setting based on the stress index measurement.[12]

Född 1957. Jag har en Atopisk Polyvalent Allergi som jag är född med. Min viktigaste dag är när jag och Michael träffades för första gången den 9/5-1990. Det var på hans jobb. Vi var ute och åt middag för första gången den 18/5-1990. Vi förlovade oss den 15/9-1991. Vi gifte oss den 3/7-1993. I övrigt får ni lära känna mig genom det jag skriver.